After knowing about atom and it’s discovery you are thinking that what is the shape/what is the structure of an atom ?
Right ? And what is inside of an atom ?
Well by my first article you understood that atom is little bit complicated but
It is also complicated by it’s inside.
Me and you nobody can see atom by naked eyes or through a simple microscope because the structure of atom is extremely small,
You have to use a powerful microscope to see atom and,
Still it is difficult to see complete structure or shape of atom.
Scientists find it’s approx shape and structure with a research of many years then they collect all knowledge about atomic structure.
When I say atom then what do you mean by an atom ? a particle or a point. Atom is a very small particle that is found in everything that is present in this universe solid, liquid and gas have it’s own atoms.
Like as water (H2O) composed of hydrogen and oxygen atoms that have combined to form water molecules . There are many examples of atom present.
There are not one atom present in anything, there are many atoms present in any type of element or matter.
Every atom has it’s different shape, size and weight but the structure of all atoms has almost same as each other.
Things that you going to know in this article –
Structure of atom
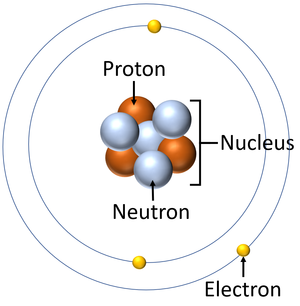
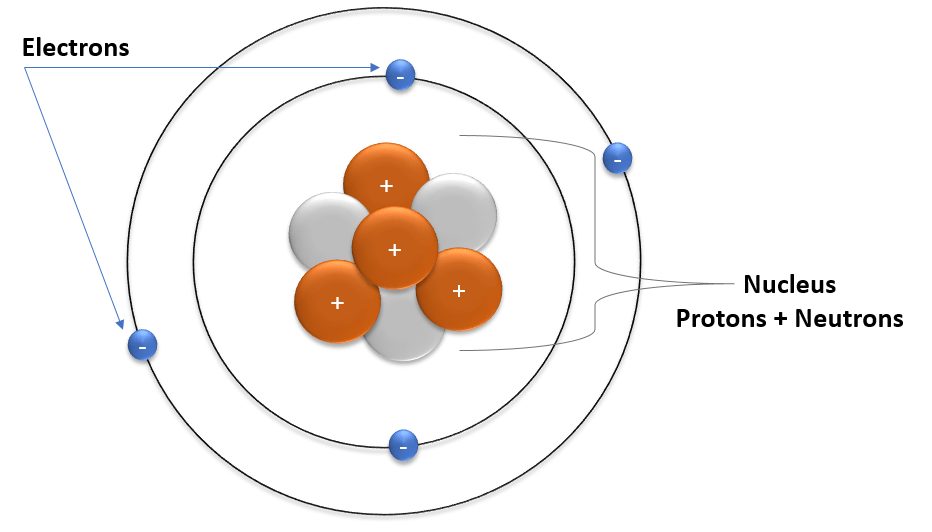
As you can see in this picture atom has a spherical shape like a ball and it is very small. The structure of atom is much typical but not difficult, it is easy to understand.
Atom is hollow from inside but at the center of atom There is a round like shape which is called atomic nucleus.
Solar System structure of atom
And in that hollow space electron revolve around the nucleus. It is like a micro solar system as in solar system all planets revolves around the same as here electrons revolves around the nucleus.
An atom consist of three basic particles – proton, neutron and electron. The nucleus (center) of the atom contains proton and neutron which have charges respectively Positive (+) and neutral (no charge) charge.
The outermost area of atom called electronic shell which contains electron which have negative (-) charge.
I told you once that inner side of atom is like as a solar system same electronic shell are the orbit where electron revolves around the nucleus in a fixed portion.
This video can help you in understanding about structure of atom.
I will give you a proper knowledge about electronic shell, electron, proton and neutron.
In a next article but now trying to give a basic knowledge of it.
Size of all atoms are different it is depending on the sizes of electrons, protons and neutrons and which type of structure of atom have.
Basically size of an average atom is about 1 × 10-10 m, a ten million of a millimeter, or 1/254,000,000 of an inch.
Despite all this empty space, solid objects do not just pass through one another.
The electron that surround all atom are negatively charged and cause atoms to repel one another, preventing atoms occupying the same space.
These intermolecular forces prevent you from falling through an object like your chair. Structure of atom is something complicated but easy to understand.
Chemical and physical Scientists are trying to make new ones every day in their labs. They are making study structure of atoms easier day by day for student like us.
Charges of atom
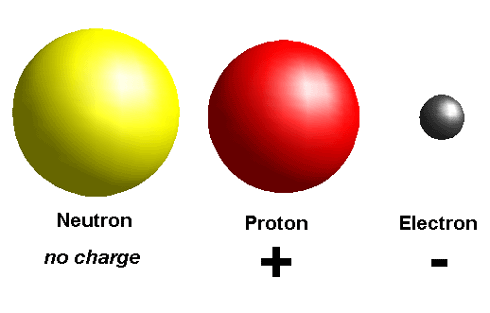
Like electricity atom also has charges, Atom is labeled with positive (+) or a negative (-) charges. Those symbols refer to the charge of atom or particle.
Have you ever heard getting a electric shock from a socket, static electricity, or lightning ? Those are all related to electric charges. Charges are also found in tiny particles of matter.
The particles that present inside of atom also carries a specific charge like as electrons always has a negative (-) charge.
The proton always has positive (+) charge. If the charge of an entire atom is neutral “0” then there are equal numbers of positive and negative charges.
Neutral atoms have equal numbers of electrons and protons. The third particle is neutron which has no (0) charge, it also known as zero charge.
Cations and Anions
The number of protons in an atom does not change, fewer or extra electrons can create some of special atom called ions. Positive charge called “Cations” and negative charge is called “Anions”.
The nucleus or the center of atom carries a positive charge because proton has positive charge and neutron has zero charge while all electrons that present around the nucleus has negative charge.
It is very amazing both different charges present in world’s smallest particle. The number of positively charged proton is perfectly charged electrons, and the charges on each are opposite but with equal magnitude.
This makes a sense as the natural state of an atom because if they held net charge, they would be much more reactive and likely wouldn’t remain in the same state for a very long before interacting with something.
Therefore in most cases the charge of an atom is the same it means zero.
Atom has electron in it’s orbit or shell and an atom is no charge but when atom fill it’s shell of electron and have some remain electron or there is deficiency of electrons
then an atom gains, then an atom holds a negative (-) charge or loss an electron to hold positive (+) charge these property of atom is called ions.
Nucleus of atom
The gaining of electron or loss of electron is a huge topic. Electron that present in atoms have their specific and fixed orbit where they revolves around the nucleus.
There is a limit of electrons in orbits or shell where limited electrons fills. Thus every atom has different limits of filling electrons in it’s orbit or shell.
Because the structure of atom is different in many atoms.
The charges of atom which presents in an atom you can clearly see in this table –
| Particles | Charges |
| Electron | -1 |
| neutron | 0 |
| proton | +1 |
As you can see atom contains three very small particles electron, proton and neutron, these are called charges of atom. The charges of atom is fully depends on these three particles.
There are approx 118 types of element in this world and universe and all atoms has different size, weight and charges of atom,
like as some atom has negative charge and some has positive charges of atom.
The most amazing thing about atom is that when an atom carries positive charges and other atom which carries negative charge attract to each other
because of charges of atom and forms a electronic bond.
which makes many other elements, molecules and compound. charges of atom is very long and deep topic modern scientists still researching on it and they are trying to make this topic easy to understand.
Conclusion
Atomic structure or structure of atom is very complicated thing and a little bit hard to understand but if you try to understand then it is become the most interesting thing of this world.
All these things that you have ever heard about it from anywhere is the hypothesis (imagination) of various scientist because it is almost impossible to get proper shape, size etc.
But truly it is one of the interesting topic not only of science but also of our daily life. All peoples should know about this, it doesn’t matter the person is student, office going person, worker etc.
Everyone should know about this. Because it is the part of our daily life.
FAQ
Basically most of atoms are balanced it means it is neutral but some atoms purely negative and positive according to their valency.
take any charge ?
If atom did not take any charges then atom is not able to be alive it becomes died but this is not practically possible.
Atom has spherical shape because of it’s nucleus and forces which is present between electrons and nucleus.
Work hard, to live large